Abstract
Apart from various HSCT forms, its outcomes are closely related to the biology and molecular genetics of the original disease as well as its status prior to the procedure. However, early in the management the ability for general prognostication in terms of HSCT planning is crucial, as this is the only curative modality available for MDS patients. Molecular markers have well established interactions with disease modifying treatment (DMT) such as HMA and particularly HSCT. Therefore, an urgent unmet need of our new molecular era has been the translation of such data into empowered prognostications tools, historically relying on hematological and cytogenetics parameters. The quest for incorporating molecular information into MDS prognostication culminated in the recent development of the IPSS-M.1 A caveat of this score is that it really projects clinical outcomes based on a virtual assumption that the patients would not be treated. Indeed, only 30% of the cohort used in the original study received DMT, and only 9% underwent HSCT, thereby limiting its applicability in a specific transplant setting. Cognizant of the instrumental clinical utility of precisely allocating MDS patients to HSCT, a procedure possibly burdened by a high rate of complications, we explored IPSS-M and transplant interactions in a large, real-life cohort of MDS patients.
With the joint efforts of an international collaborative, we accrued a total of 416 MDS patients undergone HSCT with comprehensive baseline molecular annotations and detailed clinical information on transplant-specific variables and outcomes.
Overall, the median age at MDS diagnosis was 62 years (IQR 53-67) with a 1.6 M:F ratio. According to IPSS-R, patients clustered in Very Low (5%), Low (17%), Intermediate (16%), High (27%) and Very High (35%) risk groups. In 36% of cases, no therapy with DMT had been established prior to HSCT, whereas majority (85%) of treated cases received HMA as a bridge to transplant with an overall response rate of 25%. HSCT was performed after a median time of 6.6 months (95% CI 6.1-7.3) from MDS onset. Donor choice consisted of matched related (MRD) in 20%, matched unrelated (MUD) in 60%, haploidentical in 6%, and mismatched unrelated (MMUR) in 14% of our cohort. Majority of cases underwent reduced intensity conditioning regimens (74%). As a result, peripheral blood stem cells served as graft source in 84% of cases, whereas only 16% received bone marrow. With a median follow-up time of 51 months (95% CI; 44-58 months) after HSCT, the 5-year overall (OS) and Relapse-free survival (RFS) achieved 46% and 40%, respectively. Post-HSCT relapse/leukemic progression occurred in 29% of patients at a median of 7.7 months (3.4-22.8) from the procedure, whereas 28% and 45% experienced grade II-IV acute (aGvHD) and all-grade chronic graft-vs-host-disease (cGvHD), respectively.
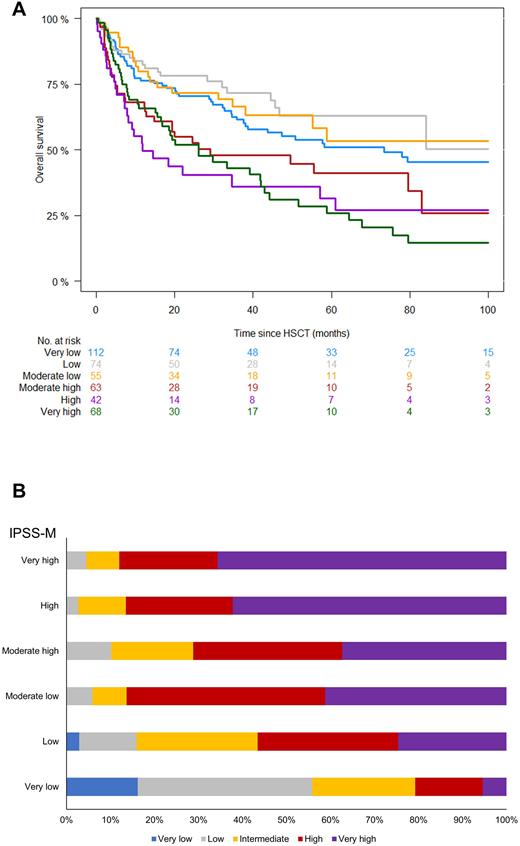
We then computed IPSS-M scores which resulted in a redistribution of risk categories as follows: Very Low (27%), Low (18%), Moderate Low (14%), Moderate High (15%), High (10%), and Very High (16%). OS at 5 years according to IPSS-M was 51%, 63%, 53%, 41%, 32%, and 26%, respectively (P<0.001; Fig.1A). Compared to IPSS-R, the incorporation of molecular information led to a significant re-stratification of patients (P<0.001; Fig.1B). Indeed, 57% of our cases carried IPSS-M molecular markers, thereby corroborating such observed differences in risk group allocation. In an attempt to assess the clinical utility of IPSS-M and its incremental benefit as opposed to IPSS-R in estimating OS/RFS outcomes in transplanted MDS, we computed Harrell's c-statistics. In terms of OS, IPSS-M resulted in a c-index of 0.583 vs 0.547 of the previous prognostication system, whereas in terms of RFS c-index was 0.584 vs 0.552, respectively.
In conclusion, the addition of molecular information represents a crucial (although still modest) advantage in prognostication of MDS undergoing HSCT. Despite being a potential useful addition for the clinical management at diagnosis, it must be acknowledged that IPSS-M inherited all the limits of its prior version, being built in a largely HSCT-naive cohort. The lower performance indexes found in ours as compared to the original study highlight that in the HSCT setting additional risk factors inherent to the transplant procedure may still hold a prognostic significance beyond the consideration of disease-specific variables.
Disclosures
Maciejewski:Alexion: Consultancy; Apellis Pharmaceuticals: Consultancy. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal